Metallographic
Atlas - example pages from the printed version
|
Go to page:
[ Previous ]
[ Index ]
[ this is the last example page]
|
1c. Physical methods of inspection
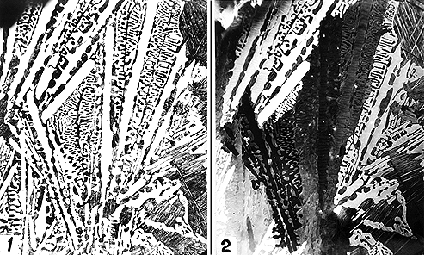
Micrograph 1. This micrograph is taken on a white cast iron and shows the eutectic mixture of cementite(white)+austenite(grey because it has later transformed to various phases which are attacked by etching). The etching reagent was nital. The cementite has not been attacked at all.
Micrograph 2. Same specimen as in picture 1 but now examined with polarized light. The polarizer and the analyzer have had crossed positions and an ordinary metallic surface would appear dark. However, cementite is not cubic. It is thus optically active which means that it rotates the plane of polarization and some light may thus pass through the analyzer. The strength of this effect is different for different orientations. One can thus distinguish between various crystals of cementite. It is evident that the fine eutectic structure has formed by sidewise growth from the long, coarse plates of cementite.

Micrograph 3. This is a specimen of pure Zn, which is a hexagonal metal. It is thus optically active and can be examined under polarized light in the polished condition. Different grains rotate the polarized light differently. We can here see that some grains contain thin regions of a different orientation. They are mechanical twins, here formed by a very light deformation.
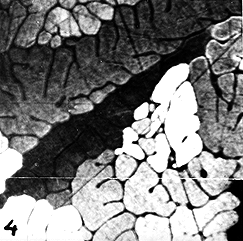
Micrograph 4. Cubic phases are optically inactive. However, sometimes they can be examined in polarized light after the kind of etching which gives a thin layer of a reaction product if that product is optically active. This micrograph shows electrolytically oxidized Al. Several fine grains with a dendritic pattern can be distinguished. Evidently, this quality of Al had some impurity which gave rise to dendritic solidification and also to many nuclei. Two scratches can be seen as thin, crossing lines. They formed during polishing and show up very well in this kind of examination.

Micrograph 5. Cubic phases may also rotate the plane of polarization if the reflection is not at right angle. Such reflection occurs on the sides of etch pits (see Fig. 1a:4). This is an Fe-C specimen containing ferrite, in addition to martensite and cementite. The specimen has been etched in a special reagent which covers the surface of ferrite with very small etch pits. They cannot be seen individually but their effect on the plane of polarization is strong. Some grains of ferrite thus appear white and some appear dark. We can here see that each grain of ferrite has two regions, one free of other phases and the other containing thin lamellae of another phase, which is cementite. That mixture should thus be regarded as very coarse pearlite. As with Figs. 1b:3 and 4, we may thus conclude that ferrite grains may start to grow alone and may later on establish collaboration with cementite.
Micrograph 6. One can apply different etching reagents one after the other and thus get different etching effects overlapping. This specimen of a low-carbon steel was first etched in nital to show ferrite(white) and pearlite(thin black regions). Then it was etched in a special reagent (Oberhoffer's etch) in order to reveal the distribution of P, present as an impurity. A dark reaction product then deposited on the areas of low P content (large black regions). It shows that P has segregated strongly during solidification. Black areas reveal the dendritic pattern of solidification. It is evident that this steel has not been deformed mechanically. The shape of the black areas shows that they represent the first, thin skeleton of dendrites. It is evident that P has had the tendency to remain in the melt, leaving the first solid to form without much P.
|
Go to page:
[ Previous ]
[ Index ]
[ this is the last example page]
|
Last change: 19 of Dec 1996
Lars Fredrik Larsson
larsf@matsc.kth.se 



